Cataract surgeons are always searching for ways to refine the procedure, improve results while preventing complications, and enhance patients’ experiences. Whether a technical or technological change, many of these adjustments help ophthalmologists to reduce unpredictability. Omidria (intracameral phenylephrine 1% and ketorolac 0.3%; Omeros) addresses a common intraoperative problem that is associated with surgical complications and enhances patients’ comfort postoperatively without requiring any changes to the routine surgical approach.
TWO BENEFITS IN ONE
Omidria is the only commercially available, FDA-approved product specifically designed both to prevent pupillary miosis and to reduce postoperative ocular pain. Diluted preoperatively in the irrigating solution (1 vial to 500 mL of BSS [Alcon]), the agent preemptively inhibits intraoperative miosis and decreases postoperative ocular pain by delivering phenylephrine and ketorolac intracamerally throughout the procedure. In effect, the agent puts the surgeon in control of these two important variables.
A stable pupillary diameter is critical for maintaining the operative field during cataract surgery. Unfortunately, it is not possible to predict with absolute certainty which patients will develop intraoperative miosis. Once the pupil begins to constrict, which often happens rapidly, the surgeon has to change technique immediately, add unplanned interventions, and sometimes, even stop the case. Pupillary constriction invariably converts a conventional cataract procedure into a complex case. The routine use of Omidria can help minimize this risk.
CLINICAL DATA
In clinical trials, the combined effects of intracameral phenylephrine to maintain mydriasis and ketorolac to prevent miosis maintained the pupil’s size over time more effectively than the use of a topical mydriatic agent alone. (Note: all patients in the studies received a preoperative topical mydriatic.) Only 4% of patients treated with Omidria had a pupillary diameter of less than 6 mm at the end of cortical cleanup compared with 23% of the placebo-treated group (P < .0001; Figure 1).1
Successful refractive cataract surgeons pay attention to patients’ experiences, but they often consider operative comfort to be the most important criterion in surgical success. Ophthalmologists may assume that patients are comfortable postoperatively. In a recent prospective, randomized, controlled study, however, about one-third of patients (30%-36%) reported moderate to severe pain after 6 hours (Figure 2).2
In FDA clinical trials, Omidria decreased postoperative pain for 10 to
12 hours after surgery. During this important period, the number of patients reporting no pain was 26% for those treated with the agent compared with 17% who received placebo
(P = .0014). There were no important complications in the treatment group compared with placebo. In addition, the agent is preservative and bisulfite free.
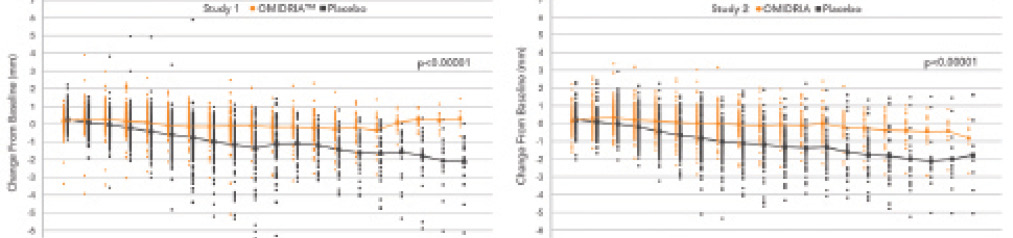
Figure 1. Intraoperative change in pupillary diameter from baseline in both Omidria- and placebo-treated patients from the clinical studies.
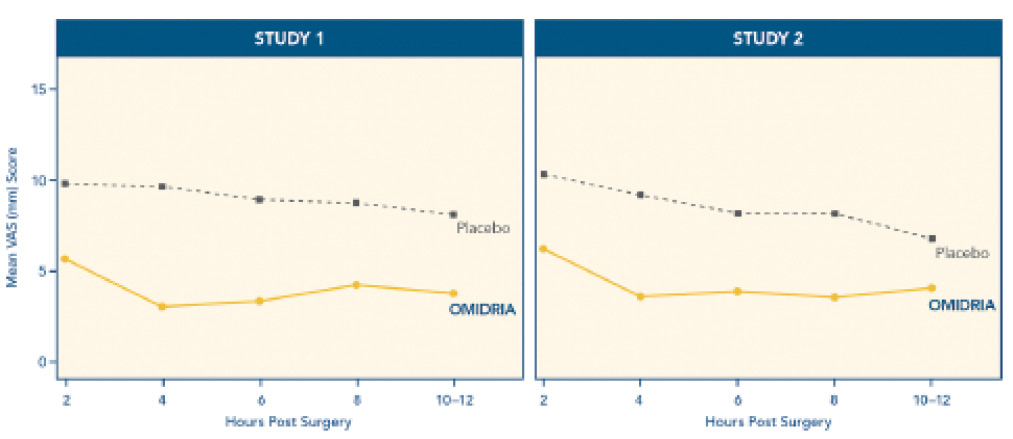
Figure 2. Important postoperative pain findings in the clinical trials.
REIMBURSEMENT
The use of Omidria does not result in additional costs to the surgeon, the surgicenter, or the Medicare patient, because the Centers for Medicare & Medicaid Services (CMS) recently conferred transitional pass-through payment status on the product. Importantly, this reimbursement decision was based on CMS’s conclusion that the agent substantially improves clinical outcomes compared with currently available treatments. Effective immediately, ambulatory surgical centers and other outpatient facilities can use a unique Healthcare Common Procedure Coding System billing code, C9447, to bill CMS for a separate payment for Omidria, outside the bundled ambulatory payment classification payment. I believe this is a true win-win!
CONCLUSION
Omidria give surgeons a unique opportunity to increase control of the procedure, improve outcomes, and enhance patients’ postoperative comfort—all without changing the procedure or its economics for Medicare patients. This agent will help ophthalmologists continue to make cataract surgery safer and more predictable, and it represents another important step forward in refractive cataract surgery.n
1. Lindstrom RL, Loden JC, Walters TR, et al. Intracameral phenylephrine and ketorolac injection (OMS302) for maintenance of intraoperative pupil diameter and reduction of postoperative pain in intraocular lens replacement with phacoemulsification. Clin Ophthalmol. 2014;8:1735-1744.
2. Porela-Tiihonen S, Kaarniranta K, Kokki M, et al. A prospective study on postoperative pain after cataract surgery. Clin Ophthalmol. 2013;7:1429-1435.
Eric D. Donnenfeld, MD
• professor of ophthalmology at NYU
• trustee of Dartmouth Medical School in Hanover, New Hampshire
• (516) 766-2519; ericdonnenfeld@gmail.com
• consultant to Omeros