Under the current standard of care, the postoperative use of topical antibiotics, steroids and a nonsteroidal anti-inflammatory drug (NSAID) after cataract surgery provides a comprehensive protective strategy to address inflammation, pain, and infection. In particular, NSAIDs have been shown to reduce inflammation, decrease the incidence of cystoid macular edema (CME), and improve visual recovery.1-3 Importantly, NSAIDs provide effective analgesia by reducing the synthesis of prostaglandins, which are mediators of pain and inflammation.4 As well, NSAIDs inhibit cyclooxygenase enzymes (COX-1 and COX-2) which play a critical role in the inflammatory process.5 Specifically targeted, NSAIDs provide precise and potent inflammatory control, leading to improved recovery and patient comfort versus a less targeted steroid. Due to the complementary and overlapping mechanisms of action, a combination of both medications is preferred to provide comprehensive anti-inflammatory coverage and reduce the risk of postoperative complications, such as CME.
Standard of care regimens have traditionally been delivered topically; however, they can be associated with several challenges, including compliance, the burden of managing multiple drops, and cost concerns. Additionally, studies have shown that approximately 93% of patients improperly administer eye drops, with more prominent misuse in older patients and those with ocular surface disease (OSD).6 In rare instances, topical NSAIDs and steroids, particularly those containing preservatives, can be toxic to the cornea, resulting in corneal melts and worsening OSD.7,8
Increasing evidence suggests that intracameral delivery of NSAIDs at the time of cataract surgery is a safe and effective alternative to postoperative drop regimens.9 Multiple studies have shown that intracameral NSAIDs reduce inflammation and the risk of macular edema, and their synergy with steroids is also well-documented.10,11 Furthermore, studies of OMIDRIA® (phenylephrine and ketorolac intraocular solution) 1% / 0.3% (Rayner) have demonstrated intraoperative pupil dilation maintenance, control of postoperative inflammation/CME, and improvement in patient comfort.10
Clinical Role of Intracameral NSAIDs
Delivery of an NSAID at the time of cataract surgery may have several advantages over postoperative use. For instance, intracameral NSAIDs help maintain pupil dilation during surgery, a critical factor for clear surgical visualization and ease of lens implantation. As well, using an NSAID intraoperatively offers immediate pain control and effective inflammation management during and after the procedure. Topical drops administered before or during cataract surgery can quickly be washed out; however, as OMIDRIA is administered in the BSS, it constantly bathes the eye with an intracameral NSAID delivered at the site of care.12
Intracameral NSAIDs can also simplify the postoperative care process, potentially reducing or eliminating the need for topical NSAID drops, thereby enhancing patient compliance and convenience.9 Intracameral delivery may have particular implications for patients with pre-existing dry eye or OSD. Many topical NSAIDs contain preservatives that can intensify surface toxicity, leading to tear film disruption, goblet cell damage, and further surface compromise.13 Thus, reducing the load of topical drops on the ocular surface substantially reduces these symptoms and improves ocular surface health.
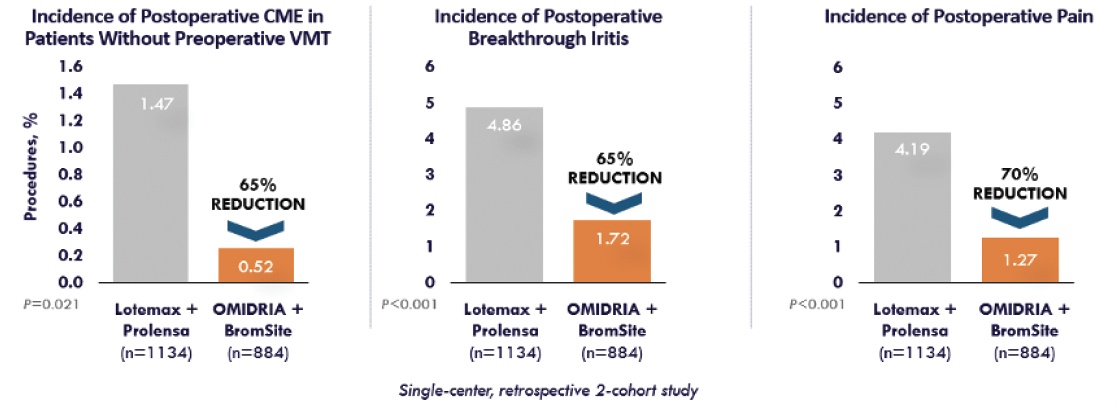
Figure. Real-world evidence demonstrates that OMIDRIA® delivers proven results.
Why OMIDRIA?
OMIDRIA’s unique formulation of phenylephrine and ketorolac is central to its role in cataract surgery. Phenylephrine is an alpha-1 agonist that maintains intraoperative pupil dilation, and consistent mydriasis may reduce the risk of intraoperative complications. For instance, small pupil size is one of the primary risk factors for posterior capsule rupture.14 Furthermore, using OMIDRIA has been shown to reduce the need for pupillary expansion devices and lessen surgical time.15,16 Meanwhile, ketorolac, a nonsteroidal anti-inflammatory, offers pain management, inflammation control, and reduction in CME postoperatively.17-19
Published phase 3 data have proven that OMIDRIA is 4 to 6 times more effective in miosis prevention than phenylephrine or ketorolac alone, with 96% of OMIDRIA-treated patients having a pupil diameter greater than or equal to 6 mm at the start of lens implantation.15,20-22 Patient experience is also improved, as data supports significantly less patient-reported postoperative pain for 10 to 12 hours with OMIDRIA versus placebo.12 Additionally, because the intracameral delivery of ketorolac in OMIDRIA provides a higher aqueous concentration than topical drops, the resulting inhibition of COX enzymes could potentially have an enhanced effect on reducing inflammatory complications, such as CME or retinal thickening.5,23
Many surgeons are now looking for effective solutions to enhance surgical outcomes and patient comfort in reduced drop or dropless surgery regimens. Intracameral steroid delivery can be achieved through the canalicular application of a slow-release steroid, and off-label options such as subconjunctival or subtenon triamcinolone. OMIDRIA is the only FDA-approved intracameral NSAID. In my experience, a dropless regimen of subconjunctival triamcinolone and OMIDRIA greatly improves patient comfort around the time of surgery in comparison to traditional protocols with drops alone.
A postoperative dropless regimen for cataract surgery that includes both an intracameral steroid and an NSAID can provide superior outcomes compared to a regimen containing only a steroid. The combination approach effectively manages and reduces inflammation, which is a common postoperative complication. Steroids are well-known for their anti-inflammatory properties, but when combined with NSAIDs, the inflammation control is more robust. NSAIDs specifically inhibit cyclooxygenase enzymes, which play a significant role in prostaglandin synthesis—a key process contributing to inflammation and pain. Studies have demonstrated that the adjunctive use of NSAIDs can lead to better control of postoperative macular edema versus steroids alone.9,11 The postoperative combination of an intracameral antibiotic, steroid, and NSAID (OMIDRIA) aligns with modern cataract surgery advancements, which aim to enhance the patient, surgeon, and clinic cataract surgery experience while controlling inflammation as well as the traditional standard of care.9
Impact on QOL and Clinical Workflow
Eliminating or reducing postoperative drops with intracameral NSAID delivery can be life-changing for patients. It has been demonstrated that 95% of patients prefer a dropless regimen when compared to traditional drops after surgery.9 In fact, many patients arrive for their cataract consultation or procedure with some trepidation regarding their assumed postoperative care and the feasibility of adhering to a complicated drop schedule. I’ve had patients with arthritis or neck injuries admit they have delayed cataract surgery because they simply did not feel they could perform the required postoperative drop regimen.
From the provider perspective, incorporating a dropless protocol into cataract surgery may have an impact on clinical workflow. This allows doctors and technicians to spend much less time answering questions about drops, educating patients on their use, coaching on adherence, and dealing with payors to determine insurance coverage for compounded drops or nonformulary drops. For example, recent data published on the use of intracanalicular dexamethasone inserts demonstrated an estimated 50% reduction in time surgeons spent educating their patients on postoperative drop use. In addition, the time surgeons spent addressing pharmacy calls regarding medication use decreased 46%.24 With this amount of time saved, clinicians can focus more on individualized postsurgical needs.
Conclusion
OMIDRIA is a core component of a traditional, less drop, or dropless cataract surgical regimen. As the only FDA-approved intracameral combination NSAID/mydriatic, it has been proven to reduce postoperative inflammation, maintain intraoperative mydriasis, improve ocular surface issues, and reduce or eliminate the need for postoperative drops.10 Because OMIDRIA is consistently infused into the eye throughout cataract surgery, inflammation is controlled before it even begins. Furthermore, high-pressure forced infusion with OMIDRIA may act as a depot, decreasing the need for postoperative NSAIDs.
While it is broadly applicable, less drop or dropless cataract surgery with OMIDRIA may be particularly beneficial for patients presenting with pre-existing OSD. If we can reduce or eliminate potentially irritating topical drops from postoperative care, we can improve both visual outcomes and comfort during the healing process so patients can enjoy their postoperative journey. In addition, by reducing postoperative complications through surgeon- versus patient-controlled delivery, we can regain valuable chair time for both physicians and staff.
In summary, while both traditional and dropless cataract surgery regimens offer pathways to improved vision, integrating OMIDRIA can result in improved patient care and surgeon experience. OMIDRIA’s unique formulation not only reduces inflammation and pain, enhances patient comfort, and maintains optimal surgical conditions, it also streamlines clinical workflows. By incorporating OMIDRIA as a core component of any cataract surgery regimen—whether standard of care, drop-light, or fully dropless—surgeons can achieve superior outcomes, enhance patient satisfaction, and reduce the burden of postoperative management. The ability of an intracameral NSAID to control inflammation before it begins, in addition to the synergistic effect with steroids, provides robust control of pain and inflammation. Thus, OMIDRIA is a preferred choice for modern cataract surgery. The evidence consistently highlights OMIDRIA’s pivotal role in elevating the standard of care, optimizing both surgical outcomes and the overall patient experience.
US-OM-2500060 10/25
1. Haddad JE, Sabbakh NA, Macaron MM, et al. NSAIDs and corticosteroids for the postoperative management of age-related cataract surgery: a systematic review and meta-analysis. Am J Ophthalmol. 2024;260:1-13.
2. Li SS, Wang HH, Wang YL, Zhang DW, Chen X. Comparison of the efficacy and safety of non-steroidal anti-inflammatory drugs and corticosteroid drugs for prevention of cystoid macular edema after cataract surgery. Int Ophthalmol. 2023;43(1):271-284.
3. Wielders LH, Lambermont VA, Schouten JS, et al. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta-analysis. Am J Ophthalmol. 2015;160(5):968-981.
4. Brune K, Patrignani P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J Pain Res. 2015;8:105-118.
5. Waterbury LD. Alternative drug delivery for patients undergoing cataract surgery as demonstrated in a canine model. J Ocular Pharmacol Ther. 2018;34(1-2):154-160.
6. An JA, Kasner O, Samek DA, Lévesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014;40(11):1857-1861.
7. Rigas B, Huang W, Honkanen R. NSAID-induced corneal melt: clinical importance, pathogenesis, and risk mitigation. Surv Ophthalmol. 2020;65(1):1-11.
8. Wolf EJ, Kleiman LZ, Schrier A. Nepafenac-associated corneal melt. J Cataract Refract Surg. 2007;33(11):1974-1975.
9. Donnenfeld ED, Hovanesian JA, Malik AG, Wong A. A randomized, prospective, observer-masked study comparing dropless treatment regimen using intracanalicular dexamethasone insert, intracameral ketorolac, and intracameral moxifloxacin versus conventional topical therapy to control postoperative pain and inflammation in cataract surgery. Clin Ophthalmol. 2023;17:2349-2356.
10. Shorstein NH, Myers WG. Dropfree approaches for cataract surgery. Curr Opin Ophthalmol. 2020;31(1):67-73.
11. Wielders LHP, Schouten JSAG, Winkens B, et al; ESCRS PREMED Study Group. European multicenter trial of the prevention of cystoid macular edema after cataract surgery in nondiabetics: ESCRS PREMED study report 1. J Cataract Refract Surg. 2018;44(4):429-439.
12. Hovanesian JA, Sheppard JD, Trattler WB, et al. Intracameral phenylephrine and ketorolac during cataract surgery to maintain intraoperative mydriasis and reduce postoperative ocular pain: integrated results from 2 pivotal phase 3 studies. J Cataract Refract Surg. 2015;41:2060-2068.
13. Kahook MY, Rapuano CJ, Messmer EM, Radcliffe NM, Galor A, Baudouin C. Preservatives and ocular surface disease: A review. Ocul Surf. 2024;34:213-224. doi: 10.1016/j.jtos.2024.08.001. Epub 2024 Aug 3. PMID: 39098762.
14. Narendran N, Jaycock P, Johnston RL, et al. The Cataract National Dataset electronic multicentre audit of 55,567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye (Lond). 2009;23(1):31-37.
15. Donnenfeld ED, Whitaker JS, Jackson MA, Wittpenn J. Intracameral ketorolac and phenylephrine effect on intraoperative pupil diameter and postoperative pain in cataract surgery. J Cataract Refract Surg. 2017;43(5):597-605.
16. Bucci FA Jr, Michalek B, Fluet AT. Comparison of the frequency of use of a pupil expansion device with and without an intracameral phenylephrine and ketorolac injection 1%/0.3% at the time of routine cataract surgery. Clin Ophthalmol. 2017;11:1039-1043.
17. Donnenfeld ED, Mychajlyszyn D, Mychajlyszyn A, Stein R. Pain control and reduction of opioid use associated with intracameral phenylephrine1.0%-ketorolac 0.3% administered during cataract surgery. J Cataract Refract Surg. 2022;48(7):759-764.
18. Jackson K, Wisely CE, Davis BM, et al. Real-world opioid prescribing after cataract surgery among patients who received intracameral phenylephrine and ketorolac 1.0%/0.3. Curr Med Res Opin. 2020;36(12):2047-2052.
19. Visco DM, Bedi R. Effect of intracameral phenylephrine 1.0%–ketorolac 0.3% on postoperative cystoid macular edema, iritis, pain, and photophobia after cataract surgery. J Cataract Refract Surg. 2020;46(6):867-872.
20. Omidria [prescribing information]. Bellevue, WA: Rayner Surgical Inc. April 2023.
21. Rayner. Data on file.
22. Lindstrom RL, Loden JC, Walters TR, et al. Intracameral phenylephrine and ketorolac injection (OMS302) for maintenance of intraoperative pupil diameter and reduction of postoperative pain in intraocular lens replacement with phacoemulsification. Clin Ophthalmol. 2014;8:1735-1744.
23. Wittpenn JR, Silverstein S, Heier J, et al; Acular LS for Cystoid Macular Edema (ACME) Study Group. A randomized, masked comparison of topical ketorolac 0.4%plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol. 2008;146:554-560.
24. Nijm L, Matossian C, Rhee MK, et al. Early real-world patient and staff experience with an intracanalicular dexamethasone insert. Clin Ophthalmol. 2024;18:1391-1401.

