Presbyopia can be a frustrating experience for patients, especially those who have been emmetropic their entire lives and suddenly face the progressive loss of near vision. Patients who have had refractive surgery may have an extra level of anxiety because they may believe their surgery is “wearing off.”
There have been few lasting surgical choices for correcting presbyopia until recently. The FDA approvals of the Kamra Corneal Inlay (AcuFocus) and Raindrop Near Vision Inlay (ReVision Optics) have made available a new approach to restoring near vision in presbyopic patients. With these approvals and at least one other corneal inlay also in development (see Corneal Inlays: Just the Facts), eye care professionals may finally have viable and potentially permanent fixes for presbyopia to offer to their patients.
KAMRA and raindrop
Although the Kamra was the first corneal inlay to gain FDA approval in April 2015, the concept behind corneal inlays dates back to the 1940s.1 The Kamra design features a small aperture in the central optical zone; the resulting pinhole effect enhances the patient’s depth of field. With the Kamra inserted in a corneal pocket in the nondominant eye, patients achieve improved near vision in that eye without having to sacrifice distance visual acuity as they would with monovision. On the other hand, there is a slight decrease in acuity in the implanted eye relative to the untouched emmetropic dominant eye.1
The ideal patient for a Kamra inlay has a slightly myopic refraction in the range of -0.50 D to -1.00 D, with a “sweet spot” around -0.75 D. The optical effect of extending the depth of field in a slightly myopic eye is to enhance near vision while also slightly improving distance acuity, as the pinhole masks much of the distance blur inherent to the small amount of myopia. In some settings, a Kamra inlay may be paired with LASIK, as the pocket created for the inlay does not necessarily interfere with flap creation; however, this is an off-label use of the device.
The Raindrop inlay has a different mechanism of action. It creates an aspheric, hyperprolate cornea that is slightly myopic in the center of the visual axis, with a decrease in the amount of myopia toward the edge of the inlay. The result is, reportedly, an optical effect similar to that of a multifocal lens, with continuous viewing zones for near, intermediate, and distancevision with less effect on distance vision compared with monovision. However, according to the FDA, in clinical trials there was a mild decrease in distance visual acuity in the Raindrop eye.
Eyes with refractions ranging from emmetropic to slightly hyperopic tend to receive the greatest benefit from the Raindrop inlay. Because the Raindrop is placed under a flap in the cornea, and because most surgeons prefer not to recut a flap, use of the Raindrop would seem to preclude the use flap-based refractive surgeries. Patients with previous LASIK surgery may have to seek other options for correction of presbyopia.
PRE- AND POSTOPERATIVE MANAGEMENT
Although the category of corneal inlays is new, many of the principles for preoperative preparation and counseling and for postoperative care are similar to those for other refractive procedures. Patients can expect to have some fluctuation in vision in the immediate postoperative period; eye dryness and mild discomfort are not uncommon. The surgery may induce some swelling of the cornea; thus, halo or glare might be noticed during the healing period. Most visual symptoms resolve, and patients should achieve their final vision by about 3 months after surgery.
CORNEAL INLAYS THE BASIC FACTS
KAMRA INLAY
Manufacturer: AcuFocus
Status: FDA approved April 2015
Design Specs
Polyvinylidine difluoride material; 6 μm thick; overall diameter 3.8 mm with a central annulus of 1.6 mm; 8,400 microperforations arranged in a pseudorandom pattern allow nutrient flow
Concept
Uses pinhole optics, by blocking peripheral rays of light to enhance depth of focus
Target Eye
Nondominant
Surgical Procedure
Implanted in a pocket at least 200 μm from the corneal surface
Clinical Trial Data
In 507 patients (age, 45-60 years) with manifest refraction spherical equivalent (MRSE) of -0.75 to +0.50 D, mean near vision improved from J8 to J2; mean distance UCVA of 20/20 was maintained, and binocular contrast sensitivity and visual fields were clinically unaffected.1,2
RAINDROP NEAR VISION INLAY
Manufacturer: ReVision Optics
Status: FDA approved June 2016
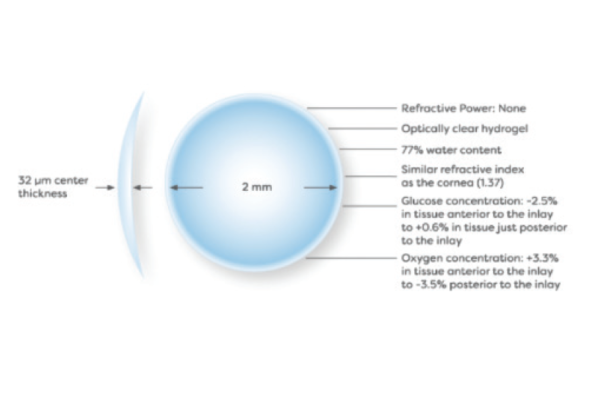
Design Specs
Hydrogel material with water content and refractive index similar to the cornea; 30 μm thick; diameter 2 mm
Concept
Creates greater depth of focus by increasing central curvature of the cornea
Target Eye
Nondominant
Surgical Procedure
Implanted under a femtosecond lasercreated flap at 30% of central corneal thickness
Clinical Trial Data
In 343 patients, average near UCVA improved by 5 lines in the treated eye at 12 months; 93% of patients achieved 20/25 near UCVA or better; intermediate UCVA improved by 2.5 lines, and distance UCVA decreased by 1.2 lines; blurred vision (1%), glare (2%), and halo (4%) were the most commonly reported side effects.3,4
FLEXIVUE MICROLENS
Manufacturer: Presbyia
Status: Approved in 42 countries; investigational in US
Design Specs
Hydrophilic acrylic polymer; available in nine refractive powers; diameter 3.2 mm, with a 1.6-mm central zone focused for distance; peripheral annulus provides focus for near
Concept
Multiple viewing zones allow the eye to focus at varying depths
Target Eye
Nondominant
Surgical Procedure
Implanted into a femtosecond laser-created corneal pocket, potentially performed in-office under topical anesthesia; investigational studies exploring use in conjunction with LASIK
Clinical Trial Data
A 3-year clinical trial has enrolled 421 emmetropic patients; trial expected to be completed in 2018.
1. Durrie D. The effect of different monovision contact lens powers on the visual function of emmetropic presbyopia patients (an American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2006;104:366-401.
2. Jalali S, Aus der Au W, Shaarawy T. AcuFocus corneal inlay to correct presbyopia using femto-LASIK. One year results of a prospective cohort study. Klin Monbl Augenheilkd. 2016;233(4):360-364.
3. Whitman J, Dougherty PJ, Parkhurst GD, et al. Treatment of presbyopia in emmetropes using a shape-changing corneal inlay: one-year clinical outcomes. Ophthalmology. 2016;123:466-475.
Kilcoyne J. Treatment of presbyopia. Paper presented at: OIS @ AAO 2015; November 12, 2015; Las Vegas, NV. Available at: http://ois.net/ois-aao-2015/presentations. Accessed April 18, 2016.
Managing preoperative dry eye disease is important for any ocular surgical procedure, but the stakes might be slightly higher in corneal implant procedures, especially for those involving the Kamra. The corneal surface is a powerful but underappreciated source of refractive influence in the eye, but, in an optical system that depends on a unilateral implant, any perception of degradation in quality of vision may be heightened because the other eye cannot compensate. In the case of Kamra patients, because they are looking through a fine pinhole in the implanted eye, any associated loss of acuity in the central zone could offset the value of the presbyopic correction. In addition, a dry corneal surface is known to affect the accuracy of biometric readings. Therefore, it is vital to detect and address existing dry eye disease during evaluation and screening to avoid treating inappropriate candidates.
IN THE PIPELINE
The Kamra and Raindrop corneal inlays represent proofs of two principles that pave the way for future development in the category of corneal inlays. However, just as each of these inlays depends on different optical designs to restore near vision, one other inlay on the horizon uses yet another optical principle to address loss of accommodation.
The Flexivue Microlens (Presbyia) is approved for use in 42 countries but does not yet have the FDA’s nod. Much like a multifocal lens, the Flexivue has multiple viewing zones with differing refractive power adds. Similar to the other inlays, it is intended for use in the nondominant eye. It creates a slight myopic shift, so the ideal candidate appears to be an individual with emmetropia to mild hyperopia. The lens is available in nine refractive powers, which could add a customizable approach to the correction of presbyopia, including exchange for a more powerful inlay as presbyopia advances. Early clinical results and data from outside the United States appear promising.
With any surgical procedure, appropriate candidacy and discussion of perioperative expectations is integral to success. Corneal inlays offer tremendous potential to permanently address a source of refractive error that can be extremely frustrating for patients, one that only promises to grow in prominence with the aging of the population. With more patients coming into the clinic, eye care practitioners would do well to understand the options so that they can refer patients appropriately.
1. Arlt E, Krall E, Moussa S, et al. Implantable inlay devices for presbyopia: the evidence to date. Clin Ophthalmol. 2015:14;(9):129-37..
2. Barraquer JI. Modification of refraction by means of intracorneal inclusions. Int Ophthalmol Clin. 1966;6(1):53-78.