
Topical therapies for the treatment of dry eye disease (DED) in the United States have for years been limited to artificial tears, steroids, and cyclosporine ophthalmic emulsion 0.05% (Restasis; Allergan). Recently, lifitegrast ophthalmic solution 5% (Xiidra; Shire) and Restasis Multidose, a preservative-free multidose formulation of the familiar anti-inflammatory drug, were introduced. New to the European market is another formulation of topical 1 mg/mL cyclosporine (Ikervis; Santen).
Cyclosporine has certainly been a beneficial therapy for DED since its FDA approval in 2002. The therapy increases goblet cell density in patients with aqueous-deficient DED.1,2 Goblet cells secrete mucus into the tear film and help to stabilize it. Cyclosporine also promotes the production by the goblet cells of an important cytokine, transforming growth factor ß2 (TGF-ß2; Figure 1), which helps to suppress inflammation.3
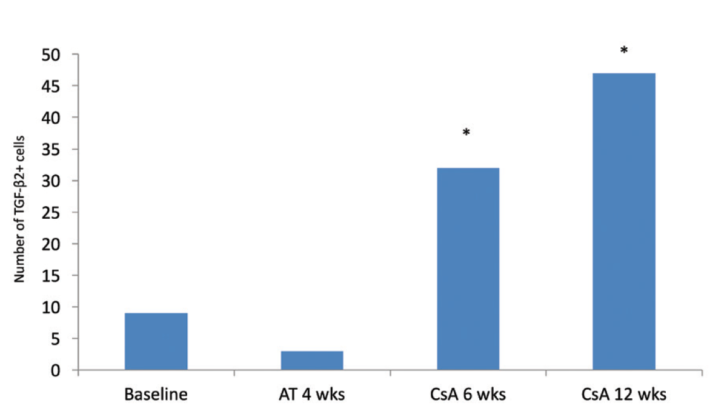
Figure 1. TGF-ß2–positive cells in temporal bulbar conjunctiva. Treatment with cyclosporine A 0.05% resulted in a significant increase in production by goblet cells of an inflammation-suppressing cytokine, TGF-ß2 (P < .001 vs baseline and vs artificial tears). Mean goblet cell density also significantly increased in the inferior bulbar conjunctiva after 6 and 12 weeks of treatment and in the temporal bulbar conjunctiva after 12 weeks of treatment compared with baseline or 4 weeks of treatment with artificial tears. Abbreviations: AT, artifical tears; CsA, cyplosporine A 0.05%. Adapted from Pflugfelder et al.3
Dosing formulations that are more convenient for patients are good additions to clinicians’ armamentariums. Likewise, it is encouraging to have a second drug for DED approved by the FDA. Lifitegrast is an integrin antagonist that binds to lymphocyte function-associated antigen 1 (LFA-1) on T cells to block the interaction of LFA-1 with intercellular adhesion molecule 1 (ICAM-1), a cell surface protein that is overexpressed in patients with DED (Figure 2). Blocking this interaction is believed to inhibit T cell activation and migration and to stop or reduce the secretion of inflammatory cytokines. Clinical studies have shown some onset of action at 2 weeks, with larger reductions in staining and dryness at 6 to 12 weeks.4
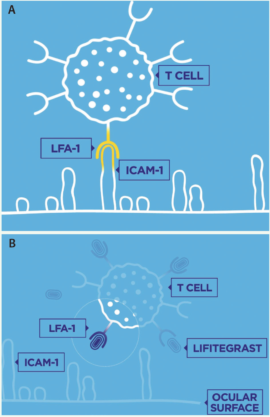
Figure 2. LFA-1 receptors on T cells interact with ICAM-1 cell surface proteins, which are overexpressed in patients with DED (A). By binding to LFA-1, lifitegrast blocks the interaction between LFA-1 and ICAM-1, inhibiting the inflammatory process (B).
STILL A NEED
It is likely that the FDA and clinical investigators took away from the lifitegrast trials some important lessons on how best to structure clinical trials in DED. These studies have been notoriously difficult to conduct because of the varied etiology of the disease, gaps in researchers’ and clinicians’ knowledge about responder characteristics, and the variability of signs and symptoms in response to environmental factors and daily activities.
Even so, there is a need for additional therapies. From the patient’s perspective, current options do not provide adequate relief of symptoms. Those who do get symptomatic relief may still have difficulty with blur on instillation, inconvenience, and compliance. From clinicians’ perspective, the available therapies do not improve ocular surface signs as much as they would like. Finally, there is probably a significant proportion of patients with DED who do not respond to these therapies because these individuals have chronic, neuropathic pain that is not at all addressed by these drugs.
NEW APPROACHES
Most of the approaches to DED therapy now in development involve some kind of sustained-release technology. The reality is that topical drops are not the ideal method for delivering a therapeutic agent to the cornea. Topical medications reach peak therapeutic concentration immediately and are then quickly washed out, rendering them ineffective.
The ideal sustained-release technology would be well tolerated, would work as well as or better than artificial tears, would be easy to use and position, and would have minimal impact on vision. This article outlines some of the therapeutic modalities in development.
Nanopolymers
There is a great deal of interest in nanopolymers, particularly those that can slowly release active ingredients over time. Kala Pharmaceuticals is investigating a mucomimetic polymer, KPI-121. These tiny particles bind to mucin in the eye and slowly release loteprednol etabonate over several days.
Phase 2 trials evaluating KPI-121 in 150 individuals with a documented clinical diagnosis of DED in both eyes reported significant improvements in conjunctival hyperemia at 2 weeks and 1 month. There were improvements in ocular discomfort, but a statistically significant difference was not achieved. There was no difference in IOP between drug and vehicle. Phase 3 trials are now underway.5
Another interesting nanoscale drug candidate is a nanomicellar formulation of cyclosporine 0.09% (Seciera; Sun Pharma). The company recently announced results of a multicenter, randomized, double-masked, vehicle-controlled phase 3 confirmatory study that included 744 patients with DED. After 12 weeks of treatment, the drug demonstrated statistically significant improvements over vehicle in Schirmer test scores and several key secondary endpoints.6
Drug-Eluting Plugs or Inserts
Noninvasive, external, sustained-release technologies in development include a drug-eluting intracanalicular depot (Dextenza; Ocular Therapeutix) that slowly releases dexamethasone and dissolves over time. This formulation is under review by the FDA for the treatment of pain after ocular surgery.7 If approved, the drug’s indications may eventually be expanded, pending trial results for DED.
Mati Therapeutics is working on a more superficial punctal plug that elutes latanoprost, but the company’s pipeline includes molecules to treat DED as well.8
Several other companies are developing sustained-release pellets or wafers similar to the hydroxypropyl cellulose ophthalmic insert Lacrisert (Bausch + Lomb) but with the goal of better-controlled release.
A phase 2 clinical trial for glaucoma treatment with a bimatoprost-eluting ring insert (Bimatoprost Ring; Allergan) has been completed, and the device was well tolerated and showed signs of efficacy.9 The Bimatoprost Ring rests on the conjunctiva under the upper and lower eyelids. The same technology could be used to deliver anti-inflammatory or other therapies to the ocular surface (see video below).
Watch It Now
In this episode of Glaucoma Today Journal Club, James Brandt, MD, and Steven Vold, MD, discuss the Bimatoprost Ring, a novel drug-delivery system for glaucoma management with other potential applications.
Better Vehicles
There are efforts to create improved drug vehicles that would still be delivered topically but would improve duration of effect, concentration of the active ingredient in the target tissues, and/or tolerability. Among these is the EyeSol technology from Novaliq. This technology is based on semifluorinated alkanes or SFAs. One compound in this family, perfluorohexyloctane, is commercially available over the counter in Europe (EvoTears; Ursapharm/Novaliq) as a nonaqueous drop that has been shown to improve comfort and staining (Figure 3).10 The product has a very low surface and interface tension that allows it to spread quickly and uniformly over the ocular surface without relying on the blink for distribution, and it has a volume that is one-fourth that of a standard eye drop, making it less prone to spillover (Figure 4).
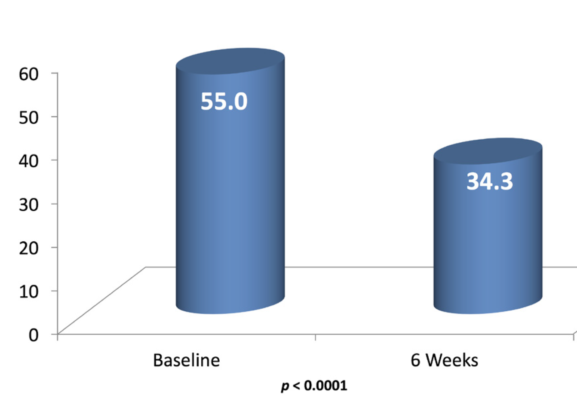
Figure 3. After 6 weeks of using a nonaqueous tear (EvoTears) four times daily, patients (n = 30) had a statistically significant improvement in mean Ocular Surface Disease Index scores.
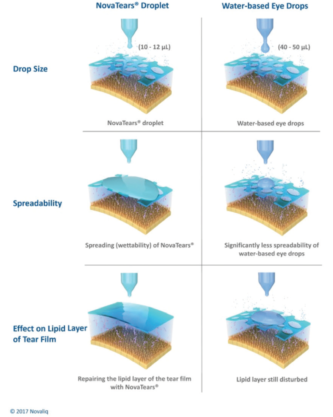
Figure 4. The NovaTears droplet is much smaller than a typical aqueous-based eye drop, which helps prevent spillover. The product spreads easily, forming a smooth, uniform layer over the tear film.
The company is using the same technology as a drug delivery system in the development of CyclASol, a preservative- and surfactant-free cyclosporine solution in a quick-evaporating SFA. Nonclinical data show that the CyclASol solution delivered cyclosporine faster and at a higher concentration to target tissues compared with Ikervis and Restasis. In a prospective, multisite, four-arm, dose-ranging study conducted in the United States, two CyclASol dosing groups (0.05% and 0.1%) showed significant improvements in corneal staining compared with vehicle at 4 months. There were improvements in corneal and conjunctival staining in as little as 14 days, and tolerability was very good, with 98% of enrolled patients completing the treatments.11
CONCLUSION
The development of effective vehicles for delivering DED therapy will continue to be important. As ongoing research suggests new potential targets at the molecular level, it may become possible to repurpose some systemic drugs for ocular uses. Many systemic drugs are poorly soluble, so vehicle technologies that keep the active ingredient stable and at the site of interest will be essential.
This is an exciting time in DED research, with a great deal of new basic science, potential new therapeutic targets and inflammatory pathways, and a number of novel approaches to treating ocular surface disease in development.
1. Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631-639.
2. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330-337.
3. Pflugfelder SC, De Paiva CS, Villarreal AL, et al. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27(1):64-69.
4. Semba CP, Gadek TR. Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease. Clin Ophthalmol. 2016;10:1083-1094.
5. Kala Pharmaceuticals announces positive results from phase 2 trial of KPI-121 in dry eye disease [press release]. Kala Pharmaceuticals. April 1, 2015. http://kalarx.com/wp-content/uploads/docs/Kala_--_dry_eye_press_release_01APR2015_en.pdf. Accessed March 15, 2017.
6. Sun Pharma announces positive topline results of confirmatory phase-3 clinical trial for Seciera for treatment of dry eye [press release]. Sun Pharma. January 4, 2017. http://www.prnewswire.com/news-releases/sun-pharma-announces-positive-topline-results-of-confirmatory-phase-3-clinical-trial-for-seciera-for-treatment-of-dry-eye-609638655.html. Accessed March 15, 2017.
7. Ocular Therapeutix announces FDA acceptance of NDA resubmission for Dextenza for the treatment of ocular pain occurring after ophthalmic surgery [press release]. Ocular Therapeutix. February 22, 2017. http://investors.ocutx.com/phoenix.zhtml?c=253650&p=irol-newsArticle&ID=2248172. Accessed March 15, 2017.
8. Mati Therapeutics. Pipeline. http://www.matitherapeutics.com/pipeline. Accessed March 15, 2017.
9. Brandt JD, Sall K, DuBiner H, et al. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: results of a phase II randomized controlled study. Ophthalmology. 2016;123(8):1685-1694.
10. Steven P, Scherer D, Krösser S, et al. Semifluorinated alkane eye drops for treatment of dry eye disease—a prospective, multicenter noninterventional study. J Ocul Pharmacol Ther. 2015;31(8):498-503.
11. Novaliq announces positive topline results of phase 2 clinical trial evaluating Cyclasol in adults with moderate to severe dry eye disease [press release]. Novaliq. January 5, 2017. http://www.novaliq.de/news/presse/. Accessed March 15, 2017.




