CASE PRESENTATION
A 16-year-old girl was recently diagnosed with keratoconus and advised to undergo corneal collagen cross-linking (CXL). The patient has been referred to you for a second opinion with respect to the diagnosis and treatment. She has no history of eye rubbing or sleeping with pressure on her eyes. Her family history is negative for keratoconus. The patient tried wearing soft contact lenses for 2 months but discontinued their use due to an inadequate improvement in her vision.
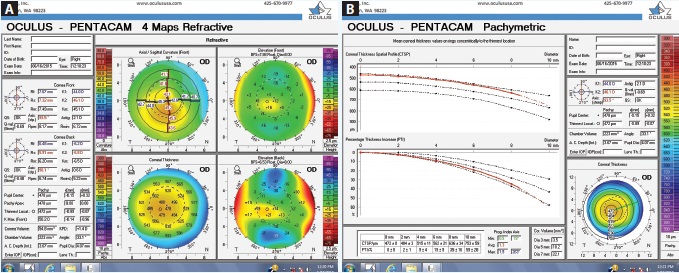
Figure 1. Tomography readings (Pentacam Comprehensive Eye Scanner) of the patient’s right eye (A and B).
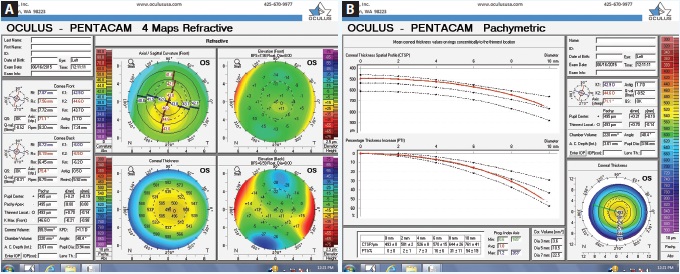
Figure 2. Tomography readings (Pentacam) of the patient’s left eye (A and B).
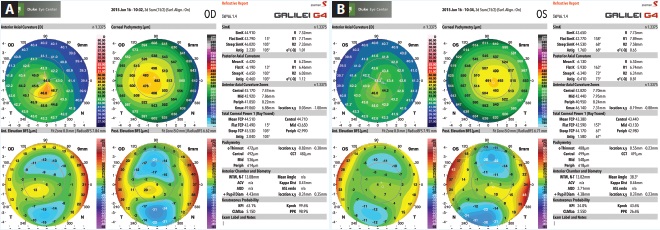
Figure 3. Measurements taken with the Galilei G4 of the patient’s right (A) and left (B) eyes.
Upon examination, her UCVA measures 20/80 OD and 20/25 OS. The patient has manifest refractions of -0.50 -0.75 × 163 = 20/40 OD and +0.25 -0.75 × 163 = 20/20- OS. Pachymetry measures 0.498 mm OD and 0.558 mm OS. Topography and tomography measurements are shown in Figures 1 to 3.
How would you advise the patient and her family with respect to her diagnosis and treatment?
—Case prepared by Alan N. Carlson, MD.

ERIC D. DONNENFELD, MD
This young woman is quite fortunate that an astute eye care practitioner has questioned the loss of BCVA in her right eye and performed the appropriate testing to diagnose corneal ectatic disease at a very early stage. She has very mild keratoconus in her right eye. The thinnest corneal pachymetry reading is 472 µm inferiorly, and this area is nasally displaced from the corneal apex, with a steepest keratometry reading of 50.2. The Galilei Dual Scheimpflug Analyzer (Ziemer Ophthalmic Systems) reveals skew deviation. The left eye presents a milder instance of forme fruste keratoconus, as defined by topographic evidence of ectatic disease without physical findings.
The first step in this case is the accurate diagnosis. The second is to halt disease progression and determine whether therapeutic intervention is indicated. Riboflavin ultraviolet CXL is a well-established treatment available internationally but one not yet FDA approved. The procedure stops the progression of keratoconus in a great majority of patients. The decision to treat this patient is her family’s after informed consent. The ophthalmologist could repeat measurements with the Pentacam Comprehensive Eye Scanner (Oculus Surgical) and Galilei in 3 months or earlier if the patient’s visual acuity worsened. If these tests showed corneal steepening, increased skew deviation, or thinning pachymetry readings, they would document disease progression and clearly indicate that treatment was needed. Alternatively, the ophthalmologist could discuss with the patient and her family the high likelihood of disease progression based on the current examination findings and state that this warrants immediate treatment.
If this patient were a member of my family, I would treat her immediately, because I would not want to put her at risk of further vision loss. The treatment options would be epithelium-on (epi-on) or epithelium-off (epi-off) CXL. Several studies have documented reduced morbidity and pain with epi-on CXL, but it may be a less efficacious approach.1 My choice in this case would be an epi-on technique that provides excellent penetration of riboflavin that turns the stroma yellow and can be directly visualized using 15 mW of energy for 5 minutes.
The third step is determining how to improve the visual acuity in the patient’s right eye. Six months after CXL, 80% of patients have improved visual acuity.2 A rigid gas permeable contact lens would be an excellent option if her postoperative visual acuity were inadequate. Alternatively, PRK with a topographic laser or the iDesign Advanced WaveScan Studio System (Abbott Medical Optics) that captured a good wavefront measurement would likely markedly improve her UCVA and BCVA.

BRAD H. FELDMAN, MD
This 16-year-old has already lost BCVA in both eyes due to irregular astigmatism from keratoconus. Her Placido disk-based topography and Scheimpflug image-based tomography clearly demonstrate classic findings of keratoconus: asymmetric inferior steepening with skewed axes, relative central corneal thinning, and focal paracentral elevation on best-fit sphere maps.
The ophthalmic literature leaves little doubt regarding the efficacy of CXL in this clinical scenario where no precipitating factors can otherwise be addressed to halt keratoconic progression.3-6 Recent randomized controlled trials confirm that treatment with riboflavin and ultraviolet light decreases corneal thinning and reduces corneal steepening and that it may improve both UCVA and BCVA.7,8 Given this patient’s age, her keratoconus would be expected to worsen for many years before eventually stabilizing.
The difficulties in this case are the patient’s age, country of residence, and possibly her family’s finances, because the FDA has not yet approved CXL. Legally, for this patient to receive treatment in the United States, she will need to enroll in an ongoing clinical trial. According to ClinicalTrials.Gov, currently, only eight studies involving investigators in just 17 states are actively recruiting children to be enrolled in CXL trials for keratoconus. These trials typically require the patient to pay for treatment (as well as for his or her travel and other costs), and in some instances, the treatment alone can cost as much as LASIK. Insurance companies are not reimbursing patients for CXL treatment. Given the challenges of geography and cost, this patient is unlikely to have access to the procedure.
In discussing treatment with the patient and her family, I would emphasize that, although her access to appropriate care is currently limited, the ophthalmology community is working with the FDA to get CXL approved and covered by insurance in the near future.

BRADLEY D. FOURAKER, MD
The first question regards the diagnosis of keratoconus in a patient with few classic symptoms. The topography and tomography do show a significant inferior/superior disparity and nonorthogonal astigmatism, with the right eye more affected than the left. There is also significant central corneal steepening and thinning in the paracentral axis. The manifest refraction does not improve the patient’s vision, in part because the refraction is likely inaccurate, especially with the tremendous change centrally. The soft contact lenses would simply follow the contour of the irregular cornea and provide modest improvement. Often, a family history is difficult to obtain in cases of keratoconus, especially without appropriate testing. The evidence, however, supports the diagnosis of keratoconus.
A previous examination is not provided, but progression of the keratoconus is to be expected, especially in a 16-year-old. The patient’s immediate visual needs should be addressed, along with the issue of likely progression. I would begin by having the patient fit with a rigid gas permeable contact lens or a specialty contact lens. If a lens with appropriate wearing time that improved vision could not be comfortably fit, then I would consider placing Intacs (Addition Technology). The intracorneal ring segments would stabilize the cornea while flattening it centrally and reducing astigmatism, improving the possibility of optical correction and decreasing disease progression.9 CXL with or without Intacs should be considered, because it is less invasive than intracorneal ring segments and may decrease keratoconic progression as well as flatten the central cornea somewhat. The CXL procedure might need to be repeated, and the patient will require optical correction postoperatively.

ROY S. RUBINFELD, MA, MD
As with many medical decisions, the key issue is the risk-benefit ratio for the patient. Early CXL clinical trials treated only patients with documented keratoconic progression, in part because the risk-benefit ratio had not yet been elucidated. In the classic Dresden protocol, the epithelium was removed in preparation for CXL, because no solutions or systems existed at the time to adequately load the stroma otherwise. Epithelial removal in CXL, however, has been associated with corneal haze, scarring, infection, perforation, and epithelial and ocular surface problems.10-12
This young girl has definite signs of keratoconus, and CXL has been shown to be extremely effective at stopping the progression of this disease. If highly effective epi-on CXL could be performed, the risk-benefit ratio for this patient would be even better than with traditional epi-off CXL. If the epithelium were not removed, then the risk of delayed epithelial healing, scarring, and infection would be reduced or eliminated. The patient’s discomfort and recovery time would likewise be improved.
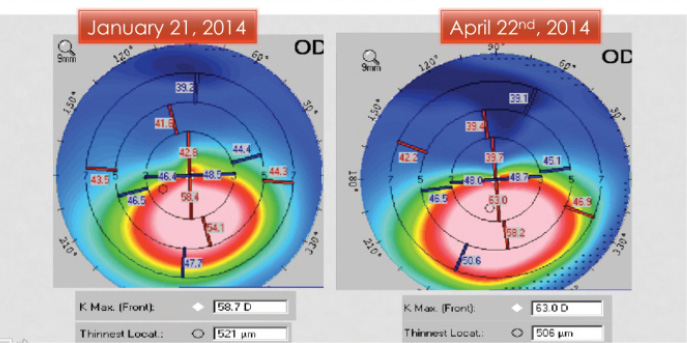
Figure 4. Significant progression of keratoconus in a 10-year-old over the course of 3 months.
I have been involved in an institutional review board-approved clinical CXL trial in the United States since 2009, and my fellow investigators and I have demonstrated excellent stromal penetration of our proprietary, patent-pending, transepithelial riboflavin formulation and system. At the 2015 American Academy of Ophthalmology Annual Meeting, we presented independent laboratory rabbit data confirming this.13,14 Some of the human trial results have been presented at international meetings during the past several years.15
This patient needs CXL. If I (or members of my family) needed this procedure, my recommendation would be the safest, most comfortable, least invasive and disruptive CXL possible, as long as that technology was effective.

WILLIAM B. TRATTLER, MD
This patient is very fortunate to have been identified so early in her disease; many patients with keratoconus are not diagnosed until their condition is more advanced. Both the Pentacam and Galilei confirm significant inferior steepening bilaterally, with the right eye more affected than the left. The patient has also experienced a loss of BCVA in both eyes, although to a greater degree in her right eye.
I would recommend moving forward with CXL as soon as possible, because her condition is likely progressive. A delay as short as 2 to 3 months in young patients can lead to a loss of BCVA and further disease progression (Figure 4).
I would recommend an epi-on technique. Although epi-off has an excellent track record, epi-on can be just as effective.16 One major difference between these two techniques is that the risk of postoperative complications would be significantly lower with epi-on versus epi-off.
In regard to improving the patient’s vision with contact lenses after CXL, my advice would be to consider a scleral contact lens for her right eye. A scleral lens would provide an excellent quality of vision, and it is also very comfortable to wear. It has been my experience that patients with early keratoconus (as is present in this patient’s left eye) who have 20/25 UCVA often feel like they see well enough without contact lenses or glasses.
I would expect bilateral epi-on CXL to stabilize this patient’s disease, and the procedure typically results in some improvement in corneal shape and vision.

DISCUSSION BY ALAN N. CARLSON, MD
As evidence emerges supporting the ability of CXL to make the cornea less elastic, I remain impressed by the frequency, duration, intensity, and vigorous pressure exerted during eye rubbing among patients with keratoconus.17 Eye rubbing is a confounding variable. It adds uncertainty to the evaluation of disease progression, the benefit of CXL, and post-LASIK keratoectasia.
In addition, there is still no consistent definition of forme fruste keratoconus. Some physicians consider it an entirely retrospective diagnosis, requiring serial chronology leading to an established diagnosis of keratoconus. Others consider it an early stage of keratoconus, particularly if the diagnosis is well established in the other eye. According to Yaron Rabinowitz, MD, “Forme fruste keratoconus presents with no slit-lamp findings or scissoring on retinoscopy, but the typical topography (asymmetric bow tie with a skewed radial axis) is once again present.”18
Of note, medical dictionaries define forme fruste very differently than how the term is used by ophthalmologists. In the former, descriptions of forme fruste include a “spontaneous arrest” in disease progression; an incomplete, attenuated, or atypical form of the disease; and an aborted course of progression.
1. Lesniak SP, Hersh PS. Transepithelial corneal collagen crosslinking for keratoconus: six-month results. J Cataract Refract Surg. 2014;40(12):1971-1979.
2. Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(1):149-160.
3. Ashwin PT, McDonnell PJ. Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol. 2010;94(8):965-970.
4. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620-627.
5. Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34(5):796-801.
6. Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet A corneal collagen cross-linking for keratoconus in Italy: the Siena Eye Cross Study. Am J Ophthalmol. 2010;149(4):585-593.
7. Wittig-Silva C, Whiting M, Lamoureux E, et al. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg. 2008;24(7):S720-S725.
8. Wittig-Silva C, Chan E, Islam FM, et al. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121(4):812-821.
9. Colin J. European clinical evaluation: use of Intacs for the treatment of keratoconus. J Cataract Refract Surg. 2006;32(5):747-755.
10. Perez-Santonja JJ, Artola A, Javaloy J, et al. Microbial keratitis after corneal collagen crosslinking. J Cataract Refract Surg. 2009;35(6):1138-1140.
11. Greenstein SA, Fry KL, Bhatt J, Hersh PS. Natural history of corneal haze after collagen crosslinking of keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010;36:2105-2114.
12. Angunawela RI, Arnalich-Montielf, Allan BD. Peripheral sterile corneal infiltrates and melting after collagen crosslinking for keratoconus. J Cataract Refract Surg. 2009;35:606-607.
13. Rubinfeld RS. Is transepithelial CXL dead? Paper presented at: The AAO Annual Meeting; November 15, 2015; Las Vegas, NV.
14. Rubinfeld RS, Trattler WB, Kanellopoulos AJ, et al. Mastering transepithelial and epithelial-off corneal collagen crosslinking for keratoconus and post-LASIK ectasia. Course presented at: The AAO Annual Meeting; November 15, 2015; Las Vegas, NV.
15. Update on U.S. clinical trials: CXL USA. ASCRS MediaCenter. http://bit.ly/1kFqMg7. Accessed November 18, 2015.
16. Trattler W, Rubinfeld R. Epithelial-on corneal crosslinking in thin corneas. Poster presented at: The AAO Annual Meeting; November 16, 2015; Las Vegas, NV.
17. Carlson AN. Keratoconus: time to rewrite the textbooks. Review of Ophthalmology. October 2009;16(10):66.
18. Rabinowitz YS. Diagnosing keratoconus and patients at risk. Cataract & Refractive Surgery Today. May 2007;7(5):85-87. http://bit.ly/1QeEYYW. Accessed December 1, 2015.
Section Editor Alan N. Carlson, MD
• professor of ophthalmology and chief, corneal and refractive surgery, Duke University Eye Center, Durham, North Carolina
• (919) 684-5769; carls009@mc.duke.edu
Section Editor Stephen Coleman, MD
• director of Coleman Vision, Albuquerque, New Mexico
Section Editor Karl G. Stonecipher, MD
• director of refractive surgery, TLC in Greensboro, North Carolina
Eric D. Donnenfeld, MD
• professor of ophthalmology at NYU
• trustee of Dartmouth Medical School in Hanover, New Hampshire
• (516) 766-2519; ericdonnenfeld@gmail.com
• financial disclosure: consultant to Abbott Medical Optics and Alcon
Brad H. Feldman, MD
• attending, Cornea Service, Wills Eye Hospital, Philadelphia
• partner, Philadelphia Eye Associates, Philadelphia
• ericdonnenfeld@gmail.com
Bradley D. Fouraker, MD
• in practice at the Brandon Cataract Center and Eye Clinic in Brandon, Florida
• (813) 681-1122; bfouraker@verizon.net
• financial interest: none acknowledged
Roy S. Rubinfeld, MA, MD
• director of Re:Vision Maryland
• clinical associate professor of ophthalmology at Georgetown University Medical Center and Washington Hospital Center in Washington, DC
• (301) 908-8091; rsrubinfeld@gmail.com; www.revisionrubinfeld.com; Twitter @MDRubinfeld
• financial disclosure: has an ownership interest and is involved in the management of CXLUSA; is an investor in and is on the board of directors of CXL Ophthalmics and CurveRight
William B. Trattler, MD
• director of cornea at the Center for Excellence in Eye Care in Miami
• (305) 598-2020; wtrattler@earthlink.net; Twitter @wtrattler
• financial disclosure: investor in CXL Ophthalmics


