CASE PRESENTATION
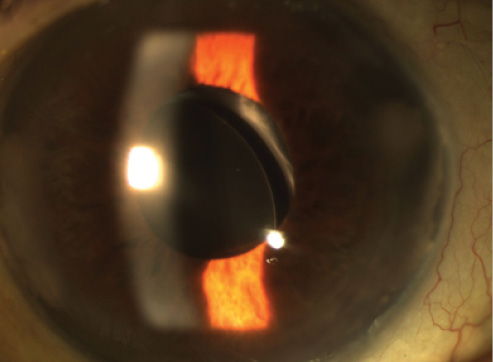
Figure. A nasally decentered IOL, clear cornea, and residual anterior capsule.
An 89-year-old woman with a history of cataract surgery and implantation of a single-piece hydrophobic acrylic IOL presents for an evaluation of recurrent uveitis-glaucoma-hyphema (UGH) syndrome. She has experienced nine episodes of hyphema during the past 12 months. Her BCVA in that eye is 20/400 due to age-related macular degeneration (AMD), and she has 20/70 BCVA in her other eye.
An examination reveals a clear cornea, a quiet anterior chamber, an open posterior capsule, and a single-piece hydrophobic acrylic IOL that is partially in the sulcus. One haptic is clearly irritating the iris. An IOL exchange is performed, and a three-piece silicone IOL (STAAR AQ2010; STAAR Surgical) is placed in the sulcus. One month after the IOL exchange, the lens is decentered, and there is some mild anterior chamber inflammation (1+ cell) but without hyphema. The patient’s visual acuity remains 20/400 in this eye.
How would you manage this case?
—Case prepared by Audrey R. Talley Rostov, MD.

GARRY P. CONDON, MD
The patient’s history is classic for a delayed onset of UGH syndrome caused by a portion of her single-piece acrylic IOL’s being left anterior to the lens capsule at the time of the original surgery. Delayed or inadequate treatment to address the problem commonly occurs if the implant is not suspected as the culprit on initial presentation. The appropriate treatment for her original UGH syndrome was, indeed, an IOL exchange for a sulcus-supported lens. If a haptic were the only portion of the IOL anterior to the capsular bag, amputation of it would have been an effective option. Most of these patients have a degree of anterior and posterior capsular fusion or a history of YAG laser capsulotomy that precludes solely using the capsular bag for repositioning or an IOL exchange. There is often sufficient residual anterior capsule, however, with a rim of well-defined capsulorhexis edge present.
Unfortunately, the sulcus-supported IOL is now subluxated. This may not be all that surprising; in my experience, many patients who have a portion of their single-piece lens outside the capsular bag have an associated history of some complexity to the original cataract procedure that kept the surgeon from comfortably ensuring the complete inclusion of the lens in the bag. These challenges include pupillary instability, a compromised capsulorhexis, or focal loss of zonular support. I suspect the last in this particular case where one of the haptics of the three-piece IOL has found an area of open zonule, resulting in subluxation and associated mild inflammation. Given the patient’s potential visual acuity of 20/400 related to the AMD, conservative therapy with topical anti-inflammatory agents might well be the most reasonable plan. On the other hand, the history and clinical photograph suggest a preserved rim of anterior capsular leaflet. There is an opportunity to potentially capture the optic of the three-piece lens by the residual capsulorhexis opening. I would consider that option if conservative measures were not helpful or if there were further subluxation with worsening visual symptoms.
Pupillary dilation and, if necessary, iris retractors have a fair chance of providing a good view of the anterior capsulorhexis. It might be possible simply to manipulate the IOL optic into the retrocapsular space with microinstruments. I would first rotate the IOL onto an axis that creates good centration. If the capsular opening were too phimotic and rigid to get the optic behind the capsule with manipulation of the optic alone, small radial cuts 180º apart could be made with microscissors to facilitate the capture. Despite violation of the capsular rim, the fibrotic nature of the anterior capsular structure will likely maintain adequate optic capture. With the lens supported by the sulcus in an axis of stability, capturing the optic would inhibit rotation back to the unstable axis. Alternatively, a single peripheral iris suture including one haptic could be placed to “lock” the IOL in a stable axis and prevent its rotation back to the unstable position. The edges of this particular vaulted three-piece silicone IOL are rounded and not at all likely to chafe the iris if the lens is stabilized.

ALAN S. CRANDALL, MD
The cause of the UGH syndrome was obvious, and removing the single-piece lens and replacing it with the STAAR AQ2010 IOL were a good choice. The decentration is unusual, because the lens has a large optic and is designed for sulcus implantation. The decentration suggests that the IOL removal was complicated by zonular issues and the lens found its way to that area. It is possible that one of the haptics was accidently placed behind the capsule, but that should have been evident at surgery by tilt or decentration. The lens can also “fly” out of the injector and potentially damage the zonules. Even though the patient has poor central vision due to AMD, I would not leave the lens in this position due to the inflammation and possible secondary glaucoma.
I would have a number of game plans, ranging from simple to complex. First, I would create two paracenteses, inject an ophthalmic viscosurgical device, and use a Kuglen hook or another push-pull device to examine the eye and see if the IOL (or haptic) were somehow damaged. In that case, I would explant the IOL and exchange it for an iris-fixated, three-piece, hydrophobic acrylic lens. If the current IOL is undamaged, one option would be to rotate it. If the capsulorhexis is centered and large enough, as they appear in the figure, and the complex is stable, the haptics could be left in the sulcus and the optic placed posterior to the capsule. If I were not comfortable with any of these scenarios, I would explant the lens, perform an anterior vitrectomy, and implant an anterior chamber IOL.

SAMUEL MASKET, MD
When performing an IOL exchange of a single-piece acrylic IOL for UGH syndrome, I prefer to capture the new optic behind the existing anterior capsulotomy in order to achieve stable fixation and prevent further posterior iris chafe. If possible, I may modify the capsulotomy with the 23-gauge vitrector. Should the capsular remnant be inadequate, however, I will invariably suture fixate the loops of the new three-piece IOL to the iris in order to ensure that the new lens implant remains stable and centered. The mild inflammatory reaction noted at present is likely the result of an unstable IOL, evidenced by the malpositioned optic edge. Although optic capture is preferable, to my mind, it is of particular importance to prevent movement of the secondary IOL when the indication for exchange is UGH syndrome. In my experience, fixation to the iris typically prevents further iris chafe, whereas a mobile IOL of any type can perpetuate the UGH syndrome.
Assuming that smoldering inflammation continues, even if treated medically, I would capture the optic posterior to the capsulotomy if possible or suture fixate the IOL loops to the iris with 10–0 polyester suture material in a McCannel fashion. Another option would be to exchange the lens for an anterior chamber IOL in order to avoid further contact with the posterior iris, but that approach is far more invasive and associated with another set of potential problems. n
Editor’s note: Douglas Rhee, MD, provided an update on UGH syndrome at this year’s annual meeting of the American Glaucoma Society. Listen to his discussion with Steven Sarkisian Jr, MD, at eyetube.net/?v=ojata.
Section Editor Lisa Brothers Arbisser, MD
• emeritus position at Eye Surgeons Associates, the Iowa and Illinois Quad Cities
• adjunct associate professor, John A. Moran Eye Center, University of Utah, Salt Lake City
Section Editor Audrey R. Talley Rostov, MD
• private practice with Northwest Eye Surgeons, Seattle
• (206) 528-6000; atalleyrostov@nweyes.com
Garry P. Condon, MD
• chairman, Department of Ophthalmology, and director, Glaucoma Division, at Allegheny Ophthalmic & Orbital Associates, Allegheny General Hospital, Pittsburgh
• (412) 359-6298; garrycondon@gmail.com
Alan S. Crandall, MD
• John A. Moran presidential professor, the Val A. and Edith D. Green presidential endowed chair of ophthalmology and visual sciences, the director of glaucoma and cataract, and the codirector of the International Division, John A. Moran Eye Center, University of Utah, Salt Lake City
• (801) 585-3071; alan.crandall@hsc.utah.edu
• financial disclosure: consultant to Alcon
Samuel Masket, MD
• clinical professor of ophthalmology, University of California, Los Angeles
• (310) 229-1220; avcmasket@aol.com