
Welcome to another edition of “Ophthalmology 360.” This column is put together by the members of CEDARS-ASPENS, a society of cornea, cataract, and refractive specialists. One of the missions of our organization is to spur innovation. This month, Dr. Milner discusses a new device to provide useful vision to patients with end-stage macular degeneration, the intraocular miniature telescope. I hope you enjoy the discussion.
— Section Editor Kenneth A. Beckman, MD

Patients with age-related macular degeneration (AMD) have benefited from therapeutic advances aimed at halting the progression of wet AMD such as antivascular endothelial growth factor agents. However, there are few options available for the patient with advanced AMD and severe vision loss. The Implantable Miniature Telescope (IMT; VisionCare Ophthalmic Technologies) offers a new option and hope to these patients.
WHAT IT IS

Figure 1. The miniature telescope is 4.4 mm in height and 13.5 mm in diameter.
The IMT is an FDA-approved miniature telescope implanted after lens extraction in one eye of a patient with bilateral end-stage macular degeneration. Patients with advanced AMD have a large central scotoma. The IMT works by magnifying the image 2.7 times, placing the image onto healthy perimacular retina, thereby “reducing the central scotoma” and improving vision, facial recognition, ambulation, and quality of life.1 The other eye, without the IMT, retains peripheral vision, which is lost in the operative eye.
In October 2014, the FDA expanded the indication of the IMT to include patients 65 years of age or older with a BCVA in each eye of 20/160 to 20/800 caused by end-stage AMD. The original indication for the IMT was for patients 75 years of age and older. The IMT is currently only indicated for phakic patients, but effective January 1, 2016, the language for the Current Procedural Terminology code (more commonly known as CPT) reads, “insertion of ocular telescope prosthesis including removal of crystalline lens or intraocular lens prosthesis.” Although the removal of an IOL and placement of the IMT is currently considered off label, the company is preparing to pursue a trial with the FDA that would broaden access to include pseudophakic patients.
HOW PATIENTS QUALIFY
Each patient must undergo a thorough preoperative evaluation to qualify for the IMT surgery. He or she must have a disciform scar or geographic atrophy with foveal involvement as a result of AMD and a cataract of grade 2 or greater. An examination must then be performed to be sure that the patient has an anterior chamber depth of at least 3 mm and an adequate cell density on specular microscopy (age 65 to < 70, ≥ 2,300 cells/mm2; age 70 to < 75, ≥ 2,000 cells/mm2; and age ≥ 75, ≥ 1,800 cells/mm2). Important contraindications to the implantation of the IMT include Stargardt macular dystrophy, an anterior chamber depth of less than 3 mm, active choroidal neovascularization, corneal stromal or endothelial dystrophies such as guttata, and zonular weakness.
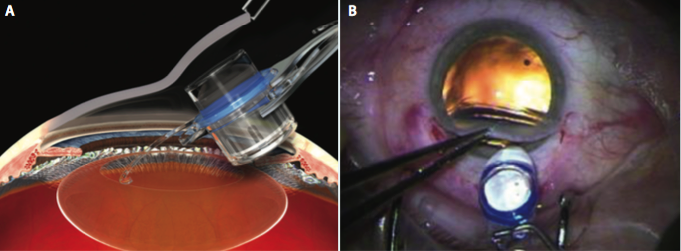
Figure 2. The IMT is inserted by lifting the cornea (A) and placing the haptics completely within the capsular bag (B).
The miniature telescope is 4.4 mm in height (approximately the size of five standard posterior chamber IOLs stacked together) and 13.5 mm in diameter (Figure 1). Compared with standard cataract surgery, the procedure for placement of the IMT is unique in that it requires a larger (12 mm) incision (similar to extracapsular cataract extraction). In addition, a 7-mm capsulorhexis is recommended, and a large amount of viscoelastic is used to protect the endothelium. Some surgeons create the large incision superiorly for placement of the IMT and a clear corneal incision temporally for the
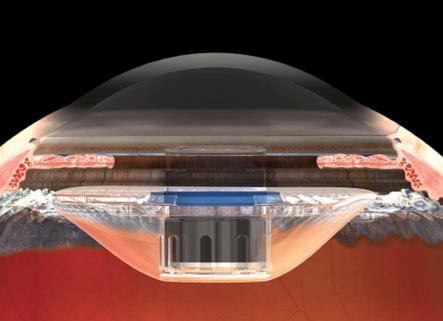
Figure 3. Once positioned properly, the device protrudes through the pupil 0.1 to 0.5 mm, creating a clearance of approximately 2.5 mm from the device to the corneal endothelium.
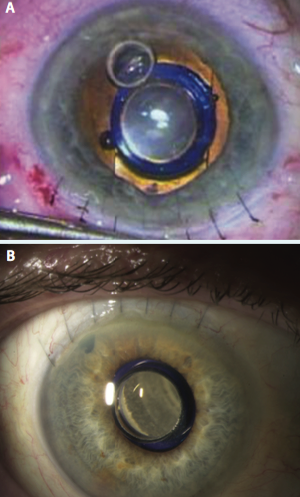
Figure 4. A peripheral iridectomy is performed (A), the viscoelastic is removed, and the incision is closed using several sutures (B).
phacoemulsification. My preference is to make a 12-mm scleral tunnel for better closure and to use a keratome within the scleral tunnel to create the incision for the phacoemulsification rather than to make two separate incisions. Once the cortical cleanup is complete, I fill the eye with viscoelastic (cohesive to fill the bag and anterior chamber and dispersive to coat the endothelium and device) and extend the incision to 12 mm. The IMT is inserted by lifting the cornea and placing the haptics completely within the capsular bag (Figure 2). Once positioned properly, the device protrudes through the pupil 0.1 to 0.5 mm, creating a clearance of approximately 2.5 mm from the device to the endothelium of the cornea (Figure 3). A peripheral iridectomy is performed, the viscoelastic is removed, and the incision is closed using several sutures (Figure 4).
The preoperative evaluation and the postoperative visual rehabilitation are just as critical to success as a well-performed procedure. Proper patient selection is essential. Once evaluated by the retina specialist and cornea/anterior segment specialist and determined to meet the criteria outlined earlier, the patient must be examined by a low vision specialist for eye selection and evaluation to determine how the patient tolerates an external telescope in one eye to simulate the monocular magnification. The patient must also see an occupational therapist to assess his or her functional goals and rehabilitation potential. This multidisciplinary approach continues postoperatively with several visits to the low vision specialist and occupational therapist for visual rehabilitation. Although the rehabilitation process may take months, I have found that the combination of a highly motivated patient and a group of dedicated specialists greatly increases the chances for an improved quality of life.
RESULTS
The published results have demonstrated a 59.5% (103 of 173) gain of 3 lines or more of BCVA in IMT-implanted eyes compared with only a 10.3% (18 of 174) gain in the fellow control eyes (P < .0001) at 2 years. Mean BCVA improved 3.6 lines for the 3× device model.2 At 5 years, the mean improvement in distance BCVA from baseline was 2.41 lines in all patients, with 2.64 lines of improvement in patients aged 65 to less than 75 years (group 1) and 2.09 lines of improvement in patients aged 75 years and older (group 2). Chronic endothelial cell density loss was consistent with that reported for conventional IOL placement.3,4 Self-reported quality-of-life scores at 12 months showed a clinically significant improvement from baseline in the majority of subscales for all age groups.1 The scores were significantly higher for group 1 compared with group 2.3 My most recent patient presented several weeks postoperatively stating that she is getting better week by week and thrilled that she can now watch TV, walk, and read better.
CONCLUSION
End-stage AMD is debilitating with central scotomas and profound vision loss. A lack of options causes many of these patients to lose hope. The IMT provides a real possibility of an improvement in visual function and quality of life.
1. Hudson HL, Lane SS, Heier JS, et al. Implantable miniature telescope for the treatment of visual acuity loss due to end-stage age-related macular degeneration: one-year results. Ophthalmology. 2006;113:1987-2001.
2. Hudson HL, Stulting, RD, Heier, JS, et al. Implantable telescope for end-stage age-related macular degeneration: long-term visual acuity and safety outcomes. Am J Ophthalmol. 2008;146:664-673.
3. Boyer D, Freund KB, Regillo C, et al. Long-term (60-month) results for the implantable miniature telescope: efficacy and safety outcomes stratified by age in patients with end-stage age-related macular degeneration. Clin Ophthalmol. 2015;9:1099-1107.
4. Bourne WM, Nelson LR, Hodge DO. Continued endothelial cell loss ten years after lens implantation. Ophthalmology. 1994;101:1014-1022.
Section Editor Kenneth A. Beckman, MD
• director of corneal services at Comprehensive EyeCare of Central Ohio in Westerville, Ohio
• clinical assistant professor of ophthalmology at The Ohio State University in Columbus and president of CEDARS-ASPENS
• (614) 890-5692; kenbeckman22@aol.com; Twitter @KenBeckmanMD
Mark S. Milner, MD
• associate clinical professor, Yale University School of Medicine, New Haven, Connecticut
• director of Corneal Services, The Eye Center of Southern Connecticut
• (203) 248-6365; eyecentermm@hotmail.com
• financial interest: none acknowledged


